Hey all! Remember the entry I've written on HPLC (High performance liquid chromatography)? I have finally managed to understand the theory behind it, thanks to the notes the biotech students have compiled in one of their modules!
So I shall give you a rough idea of how this machine works and quality control procedures we perform. Just to refresh your memory, the purpose of HPLC is an analytical process utilizing special instruments designed to:
Separate, quantify and analyze components of a chemical mixture. This analysis that is being done is based on a chromatogram that is generated after the separation process.
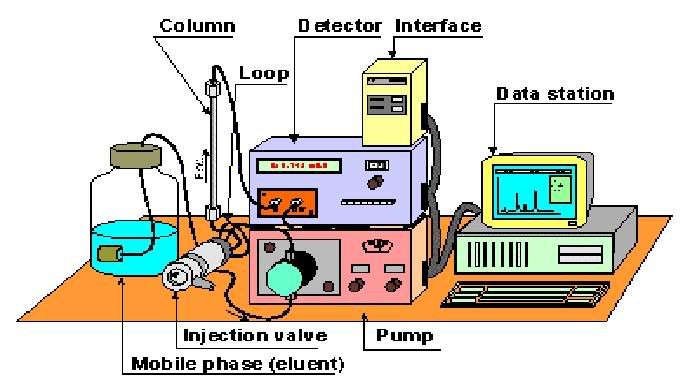
So this is how it works
1. Samples which are stored in small bottles are introduced into this compartment that contains an automatic injector. This injector works by sucking up a certain quantity of fluid to be introduced into the system.
2. The sample is passed through the system in a high pressure solvent, aka mobile phase. This solvent will then carry the samples through a column packed with sorbents. This column is known as the stationary phase.
3. Okay, so here's the gist of how it works. The chemical mixture is separated based on their polarity. Depending on their polarity, the amount of interaction between the individual components and the mobile and stationary phase differ. The individual components that have the least amount of interaction with the stationary phase or the most amount of interaction with the mobile phase will exit the column faster.
For example, if the solvent we use is polar and the column is non-polar, polar components of the mixture will usually follow the solvent, and non-polar components will adhere to the column first, only leaving the column after the polar components have exited the column. Hence, there'll be a separation of individual components based on their polarity.
4. As the analytes exit the column, they can be detected by various means. The detector shines a light through the sample, and the light is detected and saved as an electrical signal. This siganl is sent to a computer which makes a graph of the data. This graph is a chromatogram which shows peaks of active compounds in the mixture. However, this chromatogram does not tell us anything about the identity of the compound. Hence, a known standard is often used to help in the identification.
Basically, a standard refers to the active compound which you wish to identify. So after running the standard through the HPLC, a chromatogram will show up, showing only 1 major peak. By taking note of the retention time or the time it takes for the peak to show up, we'll be able to determine from the sample chromatogram which peak represents the studied active compound.
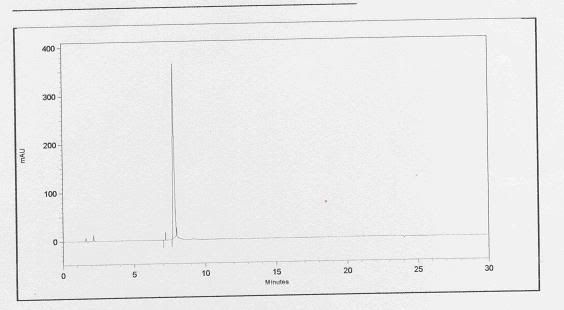
this is a chromatogram of the standard. It appears that the active compound shows up at a time frame of between 7 to 8 minutes.
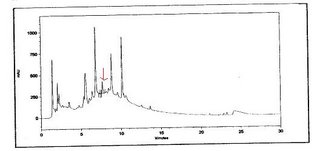
this is a chromatogram of the sample. the active compound is indicated by the arrow at a time frame of between 7 to 8 minutes.