Hmmm... These posts are long overdue...
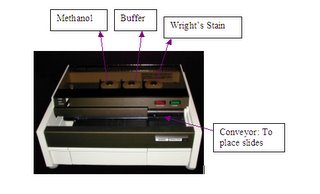
Week 2In the Immunofluorescence Lab, we were given daily tasks of recording the temperature of the lab and instruments such as the refrigerator and incubator and also the calibration of the counter analyzer. The recording of the temperature these instruments is necessary so as to ensure that the reagents used for the diagnostic tests are stored at the right temperature while the calibration of the counter analyzer is done to ensure that the results produced are accurate and consistent.
Calibration of the counter is done every morning before actual patients’ samples are analyzed. Calibration is done by running 3 blood controls of different values: Abnormal Low, Normal and Abnormal High. The control values yielded by the counter should fall within the ranges provided. If the values are out of range, the controls should be run again. To prevent false high or low results of the controls, after removing them from storage in the fridge, the controls should be left to warm up to room temperature and should be mixed well first. However, the blood control tubes should not be shaken too vigorously as it would cause bubbles. The controls should also be capped back tightly to prevent clotting of the blood and stored back in the fridge immediately after use. After the controls are run, the results would then be printed out and kept in the QC file. The counter would then be ready for use.
On Tuesday, while calibrating the counter analyzer, an unfortunate incident happened. One of the control tubes broke, spilling its content onto the floor. The medical technologists there cleaned up the blood spill according to the protocol set for Biological Spills.
Biological Spill
1) Warn co-workers.
2) Contain or cover the spill with paper towels.
3) Cover spill with 10% bleach or 1% Virkon, pouring slowly from the outside in.
4) Let the spill soak for 10 mins.
5) Pick up the soaked paper towels and dispose then in the yellow biohazard bag.
6) Inform Lab Safety officer.
Eventhough the incident was an unfortunate one & had caused a disruption in the daily workflow of the lab, I think that both, us the students and the medical technologists there learnt something from it. For us students, we got to observe an actual biological spill and the steps done to clean it up. For the med techs there, they now know that they should aliquot some of the control into another tube and keep them as a back-up so that if a similar incident were to occur, they would have another set of controls and they can continue calibrating the counter and continue with the diagnostic tests.
Week 3Besides performing the daily recording of the temperature of the lab and instruments and calibration of the counter analyzer, we learnt and performed the staining of mycoplasma and detection of Measles Antibody.
Cell lines grown in the lab are tested for mycoplasma contamination. Cell lines with passage numbers #0, 1, 2, 7 are cultured in 1 ml of medium void of any antibiotics in shell vials containing cover slips.
Bisbenzimide staining of mycoplasma
1) Make a 1/100 dilution from stock( 50 ug/ml) with absolute methanol.
2) Remove growth medium from the coverslip and wash with PBSA twice.
3) Rinse the cells once with working Bisbenzimide(0.5 ug/ml), then cover cells with about 0.5 ml of working Bisbenzimide.
4) Incubate the coverslip at 36˚C for at least 15 mins.
5) Pour off the bisbenzimide solution & rinse the cells with absolute methanol.
6) Mount and read.
Like any other tests done in the lab, positive and negative controls should also be included in each run.
In school, during Mammalian Cell Tech lectures and practicals, we learnt about how we should always practice aseptic techniques and also view our cell lines cultures daily so that if there are abnormalities in cell morphology or medium due to contamination, we could notice it immediately. We also learnt that unlike bacterial contamination, mycoplasma contamination does not cause turbidity in the medium and is thus harder to detect. Although we learnt these stuff in school, we didn’t have the opportunity to carry out the actual detection of possible mycoplasma contamination in the cell line cultures. So, I think it’s great that we get to do something related to what we have learnt.
Besides mycoplasma staining, we also performed the detection of Measles IgM and IgG.
Anti-Measles IgM IF
1) Treat serum with Gullsorb to give a final dilution of 1/10 as follows:
5 uL serum: 1 drop Gullsorb
2) Add the patient sera and positive and negative control sera to the wells on the Measles antigen slides.
3) Incubate the slides at 37˚C for 90 minutes in a moist chamber.
4) Rinse the slides twice with a stream of PBSA and immerse slides in 200 ml of PBSA and shake at 50 rpm on a shaker for 20 minutes.
5) Shake off excess PBSA from slides and add the Anti-Human IgM FITC conjugate to all wells.
6) Incubate the slides at 37˚C for 30 minutes in a moist chamber.
7) Rinse the slides twice with a stream of PBSA and immerse slides in 200 ml of PBSA and shake at 50 rpm on a shaker for 20 minutes.
8) Allow slides to air dry.
9) Mount and read slides.
The protocol for the Measles IgM and IgG tests are similar except that for the IgM test, we need to add gullsorb instead of the PBSA added in the IgG test. Gullsorb is added to dissolve the IgG found in the sera. This is important as IgG can interfere with the assay for IgM in 2 ways:
1) by competing with specific IgM for substrate binding sites
2) by forming immune complexes with rheumatoid factor which can mimic IgM, becoming a reactive site for the conjugate, thus producing an IgM false positive result.
For the first practice for the Measles Ab test, everything went smoothly and we were successful in getting the correct results. However, for the 2nd practice, we accidentally added Antigen conjugate instead of the Antibody conjugate meant for the Ab test. Haha… ya, we were very blurrrr and silly…But luckily, we realized our mistake and washed the slides thoroughly and proceeded to add the correct reagent. Despite our mistake, we still managed to get the correct results. We learnt that it is because the Ab conjugate consists of an anti-human Ab whereas the Ag conjugate contains anti-mouse Ab. Thus, the Ab did not react and interfere with assay.
Week 4Quality Assurance
External quality assessment programmes in which the laboratory participates are listed in the virology quality manual.
They consist of the following:
College of American Pathologist
i. Viral antigen survey
ii. Diagnostic immunology survey for rubella antibody
iii. Viral culture survey
iv. Viral markers genes 1 and 2 survey
v. Viral antibody survey
vi. Hepatitis C viral load survey
vii. Nucleic acid amplification survey
viii. C. trachomatis and N.gonorrhoeae (NAA) survey
WHO Immunology laboratory
National hepatitis B Quality Assurance Programme for hepatitis B surface antigen and antibody
WHO poliomyelitis Eradication Proficiency Testing for enterovirus isolation
Royal College of Pathologist of Australia Microbiology QAP
i. Antenatal serology
ii. Vaccine preventable disease serology
iii. Viral serology
iv. Trial module
National Serology reference laboratory, Australia
Centre of disease control and prevention (HIV-1) Antibody testing